By Jim Oaks
Note: This article is on batteries themselves and not on adding multiple batteries. It also does not troubleshoot charging systems.
Battery Safety:
- Always wear proper eye, face and hand protection when working with battery
- Never lean over battery while boosting, testing, or charging
- Exercise caution when working with metallic tools or conductors to prevent short circuits and arcing
- Keep terminals protected to prevent accidental shorting
- Replace any battery that has signs of damage to the terminals, case, or cover
- Install battery in a ventilated area for operation and during charging
- If your battery states to not add water to it, do not add water.
How It Works:
The modern automotive battery is a lead-acid storage design. In short, it’s an electrochemical device that converts chemical energy into electrical energy. When the battery is placed under a load, such as when the ignition is switched on, the device converts stored chemicals into electricity, and the current flows through the wires to its destination.
A standard 12-volt, lead-acid battery is made up of six cells connected in series. Each cell produces approximately 2.1 volts.
2.1 volts x 6 = 12.6 volts
The plates are formed into a plate group, which holds a number of plates of the same polarity (for instance, all positive or all negative). The like-charged plates are welded to a post strap. The plate groups are then alternated within the battery — positive, negative, positive, negative. There is usually one extra set of negative plates to balance the charge. To ensure that the different plate groups don’t touch each other, non-conductive sheets called separators are inserted between them.
The plates are made up of lead with separate plates of lead oxide, which are submerged into an electrolyte solution of about 35% sulfuric acid and 65% water. The mixture of acid and water should have a specific gravity of 1.265 at 80 degrees Fahrenheit. Specific gravity refers to the weight of a solution, with water having a reference rating of 1.000. The electrolyte in an automotive battery is therefore just slightly over one-and-a-quarter times the weight of regular water.
A chemical reaction releases electrons, allowing them to flow through conductors to produce electricity. As the battery discharges, the acid of the electrolyte reacts with the materials of the plates, changing their surface to lead sulfate. When the battery is recharged, the chemical reaction is reversed: the lead sulfate reforms into lead oxide and lead. With the plates restored to their original condition, the process may now be repeated.
Electrolyte Solution is 65% water + 35% sulfuric acid
Charge (How much power’s in the battery?):
As mentioned above, A standard 12-volt, lead-acid battery is made up of six cells connected in series. Each cell produces approximately 2.1 volts.
2.1 volts x 6 = 12.6 volts
A car battery is considered charged at 12.4 volts or higher. It is considered discharged when it’s at 12.39 volts or less. When a battery drops voltage, even a small amount, it makes a big difference. For instance, when a battery drops from 12.6 to 12.0 volts, its power drops from 100% to 25%. At 12.4 volts, a car battery is 75% charged. At 12.2 volts, it’s 50% charged.
Measuring The Voltage:
With the battery charged, open circuit voltage should be 12.6 to 12.8 volts with the engine off and all loads off. With the car running and all loads off, the voltage should be between 13.6 and 14.5 volts.
Disable the engine by pulling the fuel pump fuse. Measure the battery voltage while cranking the engine. It should read at least 9.5 to 10 volts while cranking.
If the charging voltage is low, suspect some charging system problem. But if the charging voltage is correct and the cranking voltage is low, then the battery is suspect, as you’ve already troubleshot the wiring from the battery to the starter and ground.
Some manufacturers include a built-in hydrometer to show the state of charge of the battery. This Lucite “eye” has a float immersed in the electrolyte. When the battery is charged, the specific gravity of the electrolyte increases, and the colored top of the float is visible in the window. When the battery is discharged (or if the electrolyte level is too low), the float sinks and the window appears yellow (or black). The built-in hydrometer only checks the state of charge of one cell and will not show faults in the other cells. In a non-sealed battery each of the cells can be checked with a portable hydrometer. Batteries will last longer if not stored in the discharged state. In emergencies a battery can be jump started, by the battery of another vehicle or by a hand portable battery booster (jump pack).
Charging System:
In normal automotive service the vehicle’s engine-driven alternator powers the vehicle’s electrical systems and restores charge used from the battery during engine cranking. When your engine is running, there should be 13-15 volts at the battery terminals.
When installing a new battery or recharging a battery that has been accidentally discharged completely, one of several different methods can be used to charge it. The most gentle of these is called trickle charging. Other methods include slow-charging and quick-charging, the latter being the harshest.
Battery Charging:
Both fast- and slow-charging units are used to recharge batteries. Each has its advantages. Fast chargers are the most popular. They charge batteries at a higher rate or charge–usually 40 amperes for 12-volt batteries and 70 amperes for 6-volt batteries. At this rate, fast chargers can recharge most batteries in about 1 hour. However, batteries must be in good condition to accept a fast charge. Sulfation on the plates of the battery can lead to excessive gassing, boiling, and heat buildup during fast charging. Never fast charge a battery that shows evidence of sulfation buildup or separator damage.
Trickle chargers provide low charging currents of about 5 to 15 amperes. Slow charging may require 12 to 24 hours but is the only safe way of charging sulfated batteries. In general, almost any battery can be charged at any current rate as long as excessive electrolyte gassing does not occur and the electrolyte temperature does not exceed 125°F (51.6°C). However, when time is available, slow charging is the safest and easiest method to use. In fact, many fast chargers can be adjusted to provide slow charging. The basic rule of thumb for slow charging a battery is 1 ampere for each positive plate in one cell. Use the chart below to determine the rate of charge according to the reserve capacity of the battery.
Perform charging in a well-ventilated area away from sparks and open flames.
Always be sure the charger is off before connecting or disconnecting the leads to the battery.
Remember to wear eye protection, and never attempt to charge a frozen battery.
All battery chargers have manufacturer-specific characteristics and operating instructions that must be followed.
When charging a battery in the vehicle, always disconnect the battery cables to avoid damaging the generator or other electrical components.
Maintaining A Charge During Storage – Check below about storing your battery for more charging information.
WARNING : Do not exceed the manufacturer’s battery charging limits. Also, never charge the battery if the built-in hydrometer registers clear or light yellow. Replace the battery.
Jump Starting:
When it is necessary to jump-start a car with a discharged battery using a booster battery and jumper cables, follow the instructions below to avoid damaging the charging system or creating a hazardous situation. Always wear eye protection when making or breaking jumper cable connections.
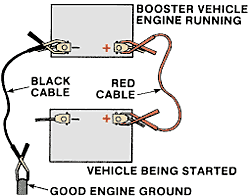
Proper setup and connections for jump-starting a vehicle with a low battery.
The following steps should be followed to safely jump-start most vehicles:
1) Make sure the two vehicles are not touching each other. The excessive current flow through the vehicle’s bodies can damage the small ground straps that attach the engine block to the frame. These small wires are designed to carry only 30 amperes. If the vehicles are touching, as much as 400 amperes may be carried through them.
2) For each vehicle, engage the parking brake and put the transmission in neutral or park.
3) Turn off the ignition switch and all accessories, on both vehicles.
4) Attach one end of the positive jumper cable to the disabled battery’s positive terminal.
5) Connect the other end of the positive jumper cable to the booster battery’s positive terminal.
6) Attach one end of the negative jumper cable to the booster battery’s negative terminal.
Another option to jump starting the vehicle is a jump pack. It’s a lot more convenient since you don’t need a second vehicle to jump start the vehicle. Jump packs plug in to a household outlet and can even be charged with an optional cord that plugs in to a cigarette lighter/DC jack.

Types of Batteries:
Lead-acid batteries for automotive use are made with slightly different construction techniques, depending on the application of the battery. The typical battery in use today is of the “flooded cell” type, indicating liquid electrolyte. AGM or Absorbed Glass Mat type batteries have no free liquid electrolyte and are gaining acceptance by consumers in SLI (Starting – Lighting – Ignition) applications. This article deals with the flooded type of car battery.
Starting Battery – The starting (cranking) or shallow cycle type is designed to deliver large bursts of energy, usually to start an engine. The SLI batteries usually have a greater plate count in order to have a larger surface area that provides high electric current for short period of time. The plates are good for lots of amps, briefly. But they break down under deep cycling–meaning lots of current drawn out of them. They should not be drawn down to under 90% capacity. Once the engine is started, they are recharged by the engine driven charging system. If you draw large amounts of current from your battery such as from winching and draw the batteries down under 90%, you could be building up damage in the battery.
Deep Cycle – The deep cycle (or motive) type is designed to continuously provide power for long periods of time (for example in a trolling motor for a small boat, auxiliary power for a recreational vehicle, or traction power for a golf cart or other battery electric vehicle). They can also be used to store energy from a photo voltaic array or a small wind turbine. They usually have thicker plates in order to have a greater capacity and survive a higher number of charge/discharge cycles. They but can be drawn down to 50% regularly without damage. The energy to weight ratio, or specific energy, is in the range of 30 Wh/kg (108 kJ/kg).
Some battery manufacturers claim their batteries are dual purpose (starting and deep cycling).
Maintenance Free Batteries – Like many things in life, the term “maintenance-free” is only partially true. Lead-acid batteries normally consume some of the water in their dilute sulfuric acid electrolyte during a normal charge-discharge cycle. It actually electrolyzes into hydrogen and oxygen and escapes as gas. So adding water periodically is necessary to keep the plates flooded. Maintenance-free batteries use a calcium alloy of lead instead of an antimony alloy, which reduces the amount of electrolysis. In addition, the amount of free-standing electrolyte above the plates is designed to be much higher in a new maintenance-free battery. This means that there’s enough electrolyte to keep the plates covered even after a few seasons of normal use. So, during the battery’s normal service life there should be no need to add water. Any abnormal electrical system condition or high ambient temperatures may boil off more than the normal amount of water, however. Adding water may extend the service life of these supposedly maintenance-free batteries.
A maintenance-free battery, similar in design to a conventional automotive battery, is really just a heavier-duty version of the same arrangement. Many of the components have thicker construction, and different, more durable materials are typically used. For example, the plate grids often contain calcium, cadmium or strontium, to reduce gassing (which causes water loss) and self-discharge. This design is called a lead-calcium battery. The heavier-duty parts ensure that fluid loss is kept to a minimum and that components have a much longer life, making it a closed system.
Picking The Right Battery:
Check your vehicle manual for the original equipment manufacturer’s recommendations for:
- Battery group size
- Cold cranking amps (CCA)
- Reserve capacity (RC) for your vehicle
Battery group size indicates the battery size that will best fit the physical dimensions of your vehicle. Many vehicles can accommodate more than one group size.
CCA is critical for good cranking ability. It’s the number of amps a battery can support for 30 seconds at a temperature of 0 degrees F until the battery voltage drops to unusable levels. A 12V battery with a rating of 600 CCA means the battery will provide 600 amps for 30 seconds at 0 degrees before the voltage falls to 7.20 V (six cells).
RC helps to power your vehicle’s electrical system if the alternator fails. It indicates the battery’s “staying power” — how many minutes the battery can supply ample power without falling below the minimum voltage needed to run your vehicle.
In general for both CCA and RC, the higher the number the better. HOWEVER, if you live in a cold climate, the CCA rating should be an important consideration in choosing a battery. Conversely, if you live in a high heat climate, you don’t need as much CCA.
If you’re looking for a deep cycle battery for marine or RV use, you must consider:
- The type of equipment to be powered
- The current (amps) needed to run the equipment
- The number of hours you’ll be using the equipment
Multiply the Amps by the Hours to determine the Amp Hours, or AH, required.
Look for a battery that will deliver the required amount of AH for the specified time and voltage. For a safety cushion, increase the number of AH by 20%. To add AH, connect batteries in parallel. To add voltage, connect batteries in a series.
Be careful of products that display only ratings such as Hot Cranking Amps (HCA) or Cranking Amps (CA)
Products that display HCA or CA ratings are tested at higher temperatures, in the case of HCA — 80 degrees — so the resulting numbers appear higher. (CAs are tested at 30°F.) Since these batteries are not as powerful as you might think, what looks like a “bargain” may end up costing you money. Make sure you are comparing apples to apples when looking at ratings. The CCA and RC are the best measure of a battery’s true power.
Check for freshness
Learn to interpret battery date codes. Or ask your retailer to make sure you purchase the “freshest” battery available. A battery that has been sitting on the shelf for extended periods can lose some of its charge and may not provide the performance you need during its first use. Long term performance probably won’t be compromised however, as the battery can be returned to its original levels of performance with either in-vehicle charging or by using an external charger.
Look for a hassle free warranty
- Is the warranty nationwide so you can obtain service wherever you are?
- How long is the free replacement period?
- After that time, will you be reimbursed for a portion of the battery’s cost on a prorated basis?
Consider value as well as price
You might want to get professional advice from your mechanic or retailer at your local parts store before you buy.
Purchase a new battery before your existing one fails.
Before you take a long trip, or when you’re having your car serviced, have your battery tested. It could save you a lot of time and money down the road.
Power Rating:
Cold Cranking Amps: Cold Cranking Amps (CCA) is critical for good cranking ability. It refers to the number of amps a battery can support for 30 seconds at 0°F until the battery voltage drops to unusable levels. For example, a 12 volt battery with 600 CCAs means the battery will provide 600 amps for 30 seconds at 0°F before the voltage falls to 7.20 volts (six cells). The higher the CCA, the more powerful the cranking ability. As the temperature drops, the cranking power required by the car increases. However, as more cranking power is used, the amount of battery power available decreases.
If you live in a cold climate, you should consider the CCA rating when choosing a battery. If you live in a very hot climate, you don’t need as much CCA.
Reserve Capacity Rating: The second standard is called reserve capacity rating. This is a warm weather rating (80 degrees Fahrenheit), which estimates the amount of time it takes the terminal voltage of a fully charged battery to dip below 10.2 (or 1.7 volts per cell) at a continuous discharge rate of 25 amps. The rating is expressed in minutes. For example, a rating of 120 means the battery will run for two hours (120 minutes) before ceasing to function.
Battery Cycles (Charge & Discharge):
A battery has two main cycles, the charge and discharge cycles.
Discharge Cycle: In the discharge cycle, a chemical reaction takes place inside the battery in which the lead (Pb) of the negative plates combines with the SO4 of the sulfuric acid to produce lead sulfate (PbSO4). In this cycle, the electrolyte becomes weaker — specific gravity lessens — and the positive and negative plates become more like one another. Since the voltage, or charge, of a battery depends on the difference between the two plate materials and the concentration of the electrolyte, and since this difference decreases during discharging, the battery loses power. To anyone who has sat in a non-starting car and cranked away as the battery grew weaker and weaker, this scenario will be immediately recognizable.
Charge Cycle: In the charge cycle, the reverse is true. Electrical current, generated by the car’s alternator, passes through the plates, forcing SO4 back into the electrolyte bath and elevating specific gravity. Voltage increases.
Fluid Level:
Formerly car batteries using lead-antimony plates would require regular watering top-up to replace water lost due to electrolysis on each charging cycle. By changing the alloying, more recent designs have lower water loss unless overcharged. Modern car batteries have reduced maintenance requirements, and may not provide caps for addition of water to the cells. If the battery has easily detachable caps then a distilled water top up may be required from time to time. Prolonged overcharging or charging at excessively high voltage causes some of the water in the electrolyte to be broken up into hydrogen and oxygen gases, which escape from the cells. If the electrolyte liquid level drops too low, the plates are exposed to air, lose capacity, and are damaged. The cells can be topped up with distilled or deionised water just above the visible plates. The sulfuric acid in the battery normally does not require replacement since it is not consumed even on overcharging.
Impurities in the water will reduce the life and performance of the battery. Manufacturers usually recommend use of demineralized or distilled water since even potable tap water can contain high levels of minerals.
Storing Batteries:
When not in active use batteries need to be monitored and periodically charged to prevent damage and retain capacity. If you remember in the section above about battery types, typical starting batteries should not be allowed to drop under 90% charge. Make sure to fully charge the battery immediately prior to storage. Clean the battery with soap and water to remove any leakage paths on the battery case due to acid or sulphation buildup on the case exterior. Dry the unit and place in a cool (below 80F if possible), dry location environment. High temperatures increase the self discharge rate. Lead-acid batteries must always be kept in a fully charged condition. Check the battery voltage with a good digital volt meter every 6 months and charge it if it falls below 12.6 volts.
Remember, newer vehicles with on-board electronics such as computers, LCD screens, game systems, GPS units, clocks, etc., require battery power to retain system memory while the vehicle is parked. If the vehicle is to be stored for long periods you should use a maintenance charger to compensate for this drain. This charger should be voltage regulated between 13.2 – 13.8 volts, 1 amp maximum. On older vehicles, without electronics, disconnect the battery cables when the vehicle is not being used for extended periods.
Float chargers like these can be used when storing batteries or during cold weather. The floating circuit maintains a full charge without overcharging and it has an automatic safety shutoff.
Float Charger
If you’re leaving the vehicle parked outside but not driving it very often, you may also want to consider a solar charger.

Solar Charger
Changing A Battery:
In most modern automobiles, the grounding is provided by connecting the body of the car to the negative electrode of the battery, a system called ‘negative ground’. The recommended practice when removing a car battery is to disconnect the ground connection first and then other terminal. This ensures that a short circuit will not occur by a wrench touching grounded engine parts while disconnecting the other terminal. Similarly, the ground should be connected last when installing a battery.
Mounting A Battery:
It’s important that you properly mount the battery. Don’t hold the battery down with bungee or and leave it loose in a battery box. Loose hold-down straps or covers allow the battery to vibrate or bounce during vehicle operation. This can have several adverse effects. It can shake the active materials off the grid plates, severely shortening battery life. It can also loosen the plate connections to the plate strap, loosen cable connections, or even crack the battery case.

Above is a battery holder designed by Blue Torch Fab

Above is a battery holder for an Optima battery.

Here is a trunk mount battery kit. Don’t just buy one of these battery boxes from a parts store and put a battery in it. Make sure you by a bracket to hold the battery down. Note the red and black bracket and long bolts to hold the battery.
Battery Maintenance:
With any type of car battery – sealed or unsealed – you must do the following to help keep it working properly:
1. Clean the cables. Disconnect the cables from the battery and clean them with a wire brush that has been doused in a home made mixture [the mixture combines one tablespoon of baking soda with one cup of water]. Use this same mixture to clean off the top of the battery; use a small and clean paint brush to apply the solution where needed. Don’t allow the solution to enter the cells of the battery.
2. Lubricate the posts. A small dab of petroleum jelly on each post will help keep your cables clean and free of corrosion longer. In addition, the jelly will make it easier for you to slip the cables back onto the battery. You can also use a corrosion preventative spray, then check the job by starting the car.
3. Check connections. Besides the connection between the cable and the posts, make sure that the battery hold down bar is securely in place. Not all cars have or need a battery hold down bar, but if yours has come so equipped, you will need to put it back in its proper place.
Battery maintenance is an easy task and one that should be performed on an annual basis. If you keep your battery properly maintained, you will extend its life and greatly reduce the chance that your battery will fail you at an inopportune time. Yes, the “maintenance free” label is incorrect, so please don’t be lulled into forgetting to do something that should never be overlooked.
Corrosion at the battery terminals can prevent a car from starting. To prevent corrosion, during regular battery service the terminals may be cleaned with a wire brush and corrosion products washed away with water. When the battery terminals are re-assembled, they are coated with Vaseline/petroleum jelly (grease is not desired) to reduce the rate of corrosion accumulation. The corrosion white powder is usually lead sulfate which is toxic by inhalation, ingestion and skin contact. It is also corrosive to the eyes and skin.
You can get an anti-corrosion spray for your battery terminals

You can get these felt rings to help prevent corrosion on the battery terminals
Battery Disposal:
Never discard an old battery in the trash. Take the old battery to a recycling center that accepts automotive batteries. Generally, part stores charge a core charge when you purchase a new battery until you turn in your old battery to them.
Battery Defects:

Common battery faults include:
- Shorted cell due to failure of the separator between the positive and negative plates
- Shorted cell or cells due to build up of shed plate material building up below the plates of the cell
- Broken internal connections due to corrosion
- Broken plates due to vibration and corrosion
- Low electrolyte
- Cracked or broken case
- Broken terminals
- Sulfation after prolonged disuse in a low or zero charged state
The primary wear-out mechanism is the shedding of active material from the battery plates, which accumulates at the bottom of the cells and which may eventually short-circuit the plates. If the battery shorts, it can get hot, boil and even explode.
Early automotive batteries could sometimes be repaired by dismantling and replacing damaged separators, plates, intercell connectors, and other repairs. Modern battery cases do not facilitate such repairs; an internal fault generally requires replacement of the entire unit.
Exploding Batteries:
Any lead-acid battery system when overcharged will produce hydrogen gas. If the rate of overcharge is small, the vents of each cell allow the dissipation of the gas. However, on severe overcharge or if ventilation is inadequate or the battery is faulty, a flammable concentration of hydrogen may remain in the cell or in the battery enclosure. Any spark can cause a hydrogen and oxygen explosion, which will damage the battery and its surroundings and which will disperse acid into the surroundings. Anyone close to the battery may be severely injured. Potentially faulty batteries can often be detected by the swollen sides where pressure has risen owing to faulty valves.
Car batteries should always be handled with proper protective equipment (goggles, overalls, gloves).
In the photo above, the internal negative connection to the plates was poor by a manufacturing defect and when the rather high starter motor current flowed, the connection failed and there was a spark inside the battery that triggered the explosion.
The battery above was being used with a generator. When the generator went to start, a loud boom was heard. It appears that when the generator went to start, a spark in the battery ignited hydrogen gas which cause it to explode.
The explosion doesn’t necessarily have to be as dramatic as the other batteries shown. You can see where the battery above is cracked at the front top corners and a piece is blown out of the top.
Frequently Asked Questions (FAQ’s):
Q – What should I consider when buying a battery?
A- SIZE: What are the dimensions of your original battery? POWER: What are the Cold Cranking Amps required to power your vehicle? WARRANTY: Automotive batteries are backed by a warranty package. Chose what is right for your vehicle’s needs.
Q – Why is it important to remove the ground wire first?
A- Before you start, always check the type of grounding system the vehicle has. If you remove the positive connector first in a negative ground system, you risk the chance of creating a spark. That could happen if the metal tool you’re using to remove the positive terminal connector comes in contact with any piece of metal on the car. If you are working near the battery when this occurs, it might create an ignition source that could cause the battery to explode. It’s extremely important to remove the ground source first.
Q- How can I tell how fresh a battery is?
A – You can usually find a small decal on the side of the battery container giving you the month and year the battery was shipped out of the plant. The letter corresponds with the month, starting with “A” for January, “B” for February, and so on. The number represents the year with “9” standing for 1999, “0” for 2000, and so on. A9, would be January, 1999. C0 would be March, 2000.( The letter “I” is skipped so the letter “M” would be December.)
Q- What does CCA mean?
A – Cold Cranking Amps is a rating used in the battery industry to define a battery’s ability to start an engine in cold temperatures. The rating is the number of amps a new, fully charged battery can deliver at 0° Farenheit for 30 seconds, while maintaining a voltage of at least 7.2 volts, for a 12 volt battery. The higher the CCA rating, the greater the starting power of the battery.
Q – What are MCA or CA rates?
A – This is a rating used to describe the discharge load in amperes which a new, fully charged battery at 32 degrees F (0C), can continuously deliver for 30 seconds and maintain a terminal voltage equal or greater than 1.2 volts per cell. It is sometimes referred to as Marine Cranking Amps or Cranking Amps.
Q – What is Reserve Capacity?
A – Reserve Capacity, (RC) is a battery industry rating, defining a battery’s ability to power a vehicle with an inoperative alternator or fan belt. The rating is the number of minutes a battery at 80 degrees F can be discharged at 25 amps and maintain a voltage of 10.5 volts for a 12 volt battery. The higher the reserve rating, the longer your vehicle can operate should your alternator or fan belt fail.
Q – What can excessive heat do to batteries?
A – Hot temperatures will deteriorate a battery’s life quicker by evaporating the water from the electrolyte, and corroding and weakening the positive grids.
Q – What is causing my battery to get hot and boil?
A – Boiling can be caused by either overcharging or from it internally shorting from one of the plates getting loose and touching another plate. Be careful because a shorting and or boiling battery can explode.
Q – My engine was running for a while and I was showing 14.2 volts at the battery terminals. A while after shutting the vehicle off, I checked battery voltage and it was only 10.3 volts. Why?
A- A battery is considered charged at 12.4 volts. Each cell has 2.1 volts. If one cell isn’t working, your 12.4 volt charged battery is only going to be capable of 10.3 volts (12.1 volts – 2.1 volts = 10.3 volts)
Terms:
Ampere-hours (A·h) is the product of the time that a battery can deliver a certain amount of current (in hours) times that current (in amps), for a particular discharge period. This is one indication of the total amount of charge a battery is able to store and deliver at its rated voltage. This rating is rarely stated for automotive batteries.
Cranking amps (CA), also sometimes referred to as marine cranking amps (MCA), is the amount of current a battery can provide at 32°F (0°C). The rating is defined as the number of amperes a lead-acid battery at that temperature can deliver for 30 seconds and maintain at least 1.2 volts per cell (7.2 volts for a 12 volt battery).
Cold cranking amps (CCA) is the amount of current a battery can provide at 0°F (−18°C). The rating is defined as the current a lead-acid battery at that temperature can deliver for 30 seconds and maintain at least 1.2 volts per cell (7.2 volts for a 12-volt battery). It is a more demanding test than those at higher temperatures.
Hot cranking amps (HCA) is the amount of current a battery can provide at 80°F (26.7°C). The rating is defined as the current a lead-acid battery at that temperature can deliver for 30 seconds and maintain at least 1.2 volts per cell (7.2 volts for a 12-volt battery).
Reserve capacity minutes (RCM), also referred to as reserve capacity (RC), is a battery’s ability to sustain a minimum stated electrical load; it is defined as the time (in minutes) that a lead-acid battery at 80°F (27°C) will continuously deliver 25 amperes before its voltage drops below 10.5 volts.
Battery Council International group size (BCI) specifies a battery’s physical dimensions, such as length, width, and height. These groups determined are by the Battery Council International organization.
SLI battery (Starting – Lighting – Ignition) is a typical battery used to power the starter motor, the lights, and the ignition system of a vehicle’s engine.
Peukert’s Law expresses the fact that the capacity available from a battery varies according to how rapidly it is discharged. A battery discharged at high rate will give fewer amperehours than one discharged more slowly.
